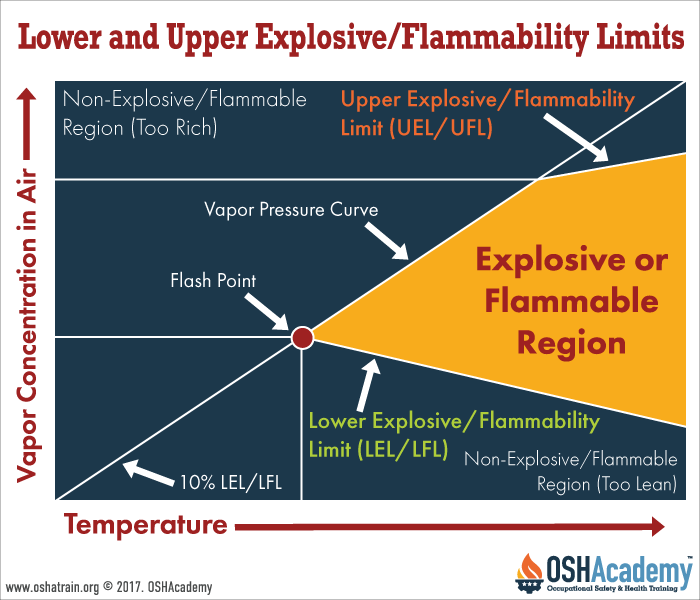
Lower Explosive/Flammability Limits (LEL/LFL)
The lower explosive limit, or LEL, is the lowest atmospheric concentration of fuel in the fuel-air mixture at which a gas or vapor can explode. The lower flammable limit, or LFL, is the lowest concentration at which the gas or vapor will burn. Fuel concentrations below the LEL and LFL will not explode or burn because there is not enough fuel in the mixture and is considered too "lean."
For example, the lean flammability limit for Jet A (aviation kerosene) in air at sea level is a concentration (by volume or partial pressure) of about 0.7%. The rich flammability limit is about 4.8% by volume or partial pressure. Flammability limits are not absolute but depend on the type and strength of the ignition source.
Upper Explosive/Flammability Limits (UEL/UFL)
The highest atmospheric concentration of a gas or vapor in the fuel-air mixture that can explode is called the upper explosive limit, or UEL. The upper flammability limit, or UFL, is the maximum fuel concentration above which the mixture will not burn. Above this concentration, the mixture will not explode or burn because it has too much fuel and is considered too "rich."
The composition of a fuel vapor and air mixture can change over time and may fluctuate within a confined space. Fluctuations occur because the fuel-air mixture moves around the space, particularly when people or other things create air currents that disturb the atmosphere. Consequently, the mixture is not uniformly distributed within the space.
Flammability Range
Gases or vapors can only be explosive or flammable between their LEL/LFL and UEL/UFL. This is called the explosive/flammable range.
Substances with a wide explosive/flammable range are more hazardous than substances with a narrow explosive/flammable range. However, any concentration of combustible gas or vapor should be a serious concern in a confined space.
Workers should be especially careful when ventilating a space containing a gas or vapor above its UEL/UFL. Ventilation will first bring the gas or vapor within its explosive/flammable range to reduce the concentration below the LEL. When it does, the possibility of an explosion or fire exists.
The diagram to the right shows the relationship among the lower and upper explosive/flammability limits, explosive/flammable and non-explosive/flammable regions, flash points, and the vapor pressure curve. It also reveals what happens to a vapor/air mixture as concentrations and temperatures vary.
Knowledge Check Choose the best answer for the question.
2-5. You are conducting an initial evaluation of a site contaminated with automobile gasoline (petrol). The NIOSH Pocket Guide indicates that gasoline has an LEL of 1.4% and a UEL 7.6%. Which concentration is least likely to be explosive?
You forgot to answer the question!